How the 4 drivers of bronchiectasis contribute to disease progression 1
How the 4 drivers of bronchiectasis contribute to disease progression 1
Bronchiectasis has been characterized in scientific literature as a vicious cycle or vortex consisting of 4 primary drivers. 1,2
Chronic airway infection
Lung destruction
Chronic airway inflammation, primarily neutrophilic
Impaired mucociliary clearance
Each primary driver can lead to the worsening of the others and contribute to progressive lung damage and damaging exacerbations. 1,3,6
Expert commentary: The vicious vortex of bronchiectasis
Let me just talk a little bit about bronchiectasis and its pathophysiology. There is an impairment of its normal mucociliary clearance function, and when that is abnormal, it becomes a ripe place for opportunistic bacteria to set up shop. With that growth of bacteria then comes an influx of inflammation. That inflammation, although it’s necessary to combat the bacteria, unfortunately is also injurious to the normal tissues. And so, you then get this injury to the airway. It again more adversely affects the mucociliary clearance, and thus begins the cycle. It just keeps feeding upon itself. Historically, that’s what led to the terms, the vicious cycle of pathophysiology of bronchiectasis. That was the traditional model, but what we’ve learned is it’s not one step feeds the next. Each one feeds the others. So that impaired mucociliary clearance not only allows for mucus retention, it also prevents the inflammation from being cleared. It incites more inflammation for coming in. The inflammation is causing injury to the airway walls, but so are products from the bacteria. And so, it wasn’t just a one step feeds the other, each is feeding the others, so that’s why we called it a vortex.
Each of these processes are interconnected, and one often begets the other. So the bacterial infection doesn’t just cause direct injury, but it incites inflammation, and the white blood cells release products, such as serine proteases, that will cause more inflammation and damage. One example is that these serine proteases will directly induce increased secretion of mucus, which may have been the body’s attempt to respond to this inflammation and injury, but can result in more mucus plugging. Therefore, more difficulty in clearing the secretions, and you get this interconnection.
When we talk about the vicious vortex, when you have inflammation, it’s important to try to treat that because if inflammation is not well controlled, it can lead to further bronchiectasis and furthering lung destruction and kind of perpetuates that vortex into worsening lung function overall. And managing one step still may lead to perpetuation of bronchiectasis and inflammation and worsening lung destruction. So even if you try to intervene on one of those steps, for example, we know that airway clearance is an important management strategy, but it may not be enough to disrupt the cycle or vortex.
Yeah, I think antibiotics are another example. Just treating someone for their bacterial infection does not necessarily affect further lung destruction, because there may be other mechanisms that underlie the inflammation that results in destruction.
A current area of investigation in bronchiectasis is the role of neutrophils and the activity of neutrophil-derived enzymes 2
Bronchiectasis mechanism of disease
Bronchiectasis is a chronic lung disease marked by permanent, abnormal dilation of the bronchi and chronic airway inflammation. While there are various etiologies of bronchiectasis such as infection, COPD, asthma, and GERD, in many patients the etiology cannot be identified (idiopathic bronchiectasis). Common symptoms of bronchiectasis include a chronic cough with sputum production, dyspnea, fatigue, and hemoptysis. Many patients suffer from repeated exacerbations, generally defined by an increase in daily respiratory symptoms and potential change in therapy. Increased frequency of exacerbations can be associated with disease progression and reduced quality of life. A diagnosis of bronchiectasis requires both the presence of signs and symptoms such as chronic productive cough or history of exacerbations, and radiological evidence, often via a high-resolution computed tomography scan.
Bronchiectasis pathophysiology has been described in literature as a "vicious cycle" or "vicious vortex," consisting of 4 interconnected components: abnormal mucus production and mucociliary clearance, where thick mucus becomes trapped in the airways, which renders the airway more susceptible to chronic infections. The inflammatory response is complex. It is primarily neutrophilic, but also involves a network of cytokines and other inflammatory cells, including macrophages, eosinophils, and lymphocytes. Collectively, these add to airway damage and lung destruction. Within this self-perpetuating process, each component can contribute to the worsening of the others and thereby furthers the progression of disease over time.
Neutrophilic inflammation plays an important role in the development and progression of bronchiectasis.
Neutrophils are immune cells with antimicrobial properties. As neutrophils mature in the bone marrow, neutrophil serine proteases (NSPs) are activated by dipeptidyl peptidase-1 (DPP-1) and packaged into azurophil granules. Once matured, neutrophils enter the bloodstream, where, upon stimuli such as an infection, they are transported along a chemotactic gradient and exit the bloodstream at the site of infection.
Neutrophils exert several host defense mechanisms, including antimicrobial functions such as the release of cytokines to recruit other immune cells, engulfment of microbes via phagocytosis, degranulation to release antimicrobial NSP molecules like neutrophil elastase, and formation of neutrophil extracellular traps (NETs). Once neutrophils have executed their antimicrobial processes, they are eliminated via apoptosis to avoid further damage.
However, in bronchiectasis, chronic infection and changes in the airway environment can lead to neutrophil dysregulation. This results in amplified numbers of neutrophils that not only survive longer but also lead to increased NSP activity and NET formation. NE activity and NETs have been associated with bronchiectasis disease severity and progression. This unresolved inflammatory process contributes to tissue damage, impaired mucociliary clearance, impaired bacterial phagocytosis, and killing resulting in airway damage.
Targeting the underlying neutrophilic inflammation associated with bronchiectasis continues to be an unmet need, and a comprehensive treatment approach is desirable. Current treatment efforts are targeted towards other components of the disease, such as antibiotics to treat chronic bacterial infection and long-term mucoactive therapies and airway clearance techniques to improve mucociliary clearance and reduce airway damage. However, individual treatments may not halt disease progression, as chronic neutrophilic inflammation and airway destruction can still be sustained by other stimuli.
Therefore, a comprehensive multimodal treatment approach that addresses all 4 interrelated components of bronchiectasis may help to reduce the frequency of exacerbations in bronchiectasis patients and improve their quality of life.
Management in bronchiectasis has been focused on combating infection, improving airway clearance, and mitigating the impact of chronic lung disease. 7 Neutrophils and neutrophil-derived enzymes such as neutrophil serine proteases are key drivers of airway inflammation in bronchiectasis; however, there are limited treatments to adequately address chronic airway inflammation in patients. 8-10

Neutrophils normally serve as the first line of defense against a range of pathogenic infections, but during bronchiectasis, changes in the airway environment cause neutrophils to become dysregulated, which leads to longer survival and release of excessive neutrophil serine proteases, including neutrophil elastase. 9-12
The overactivity of neutrophil elastase contributes to chronic inflammation, concomitant tissue damage, and an increased risk of future exacerbations. 13-15
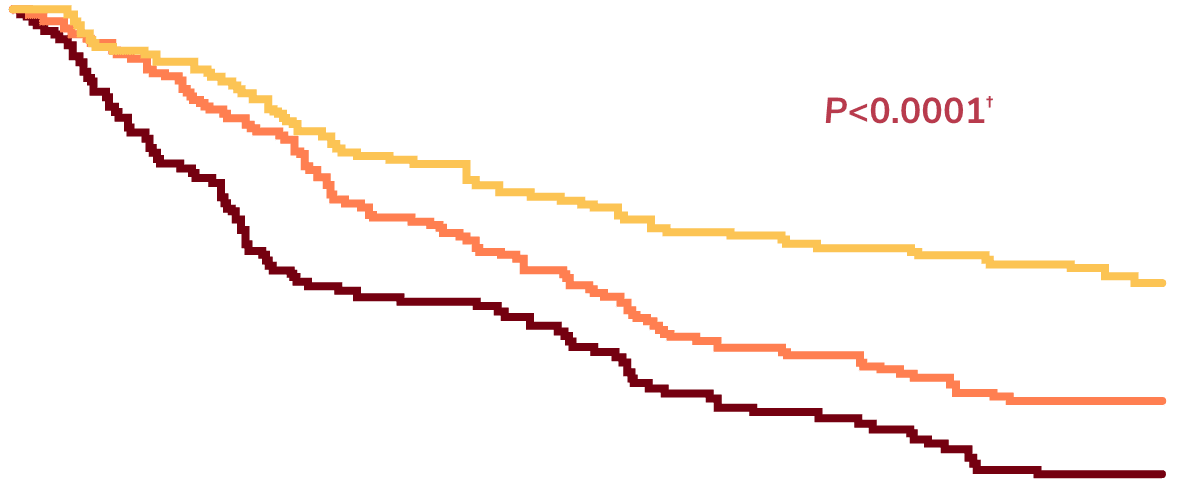
Elevated neutrophil elastase is correlated with worse patient outcomes 15
Data are from a single-center prospective observational study of 381 patients with bronchiectasis in the United Kingdom who provided sputum samples sufficient for measurement of neutrophil elastase activities. 15
Sputum neutrophil elastase level:
Sputum neutrophil elastase levels generally are not tested in routine practice, as sputum tests for neutrophil elastase are not commonly available. 15
Time to next hospitalization for severe exacerbation over 36 months. 15
Comparison of high-neutrophil elastase group vs low-neutrophil elastase (reference) group by log-rank test. 15
Source: Reprinted with permission of the American Thoracic Society. Copyright © 2025 American Thoracic Society. All rights reserved. Chalmers JD, Moffitt KL, Suarez-Cuartin G, et al. 2017. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med. 195(10):1384-1393. The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.